Check out our σ-Bond insertion reactions of two strained diradicaloids, published in Nature!
Read MoreCheck out our Solution to the Anti-Bredt Olefin Synthesis Problem published in Science!
Read MoreCheck out our Strain-Promoted Reactions of 1,2,3-Cyclohexatriene and Its Derivatives published in Nature!
Read MoreCheck out our Elucidating the Molecular Programming of a Nonlinear Non-Ribosomal Peptide Synthetase Responsible For Fungal Siderophore Biosynthesis published in Nature!
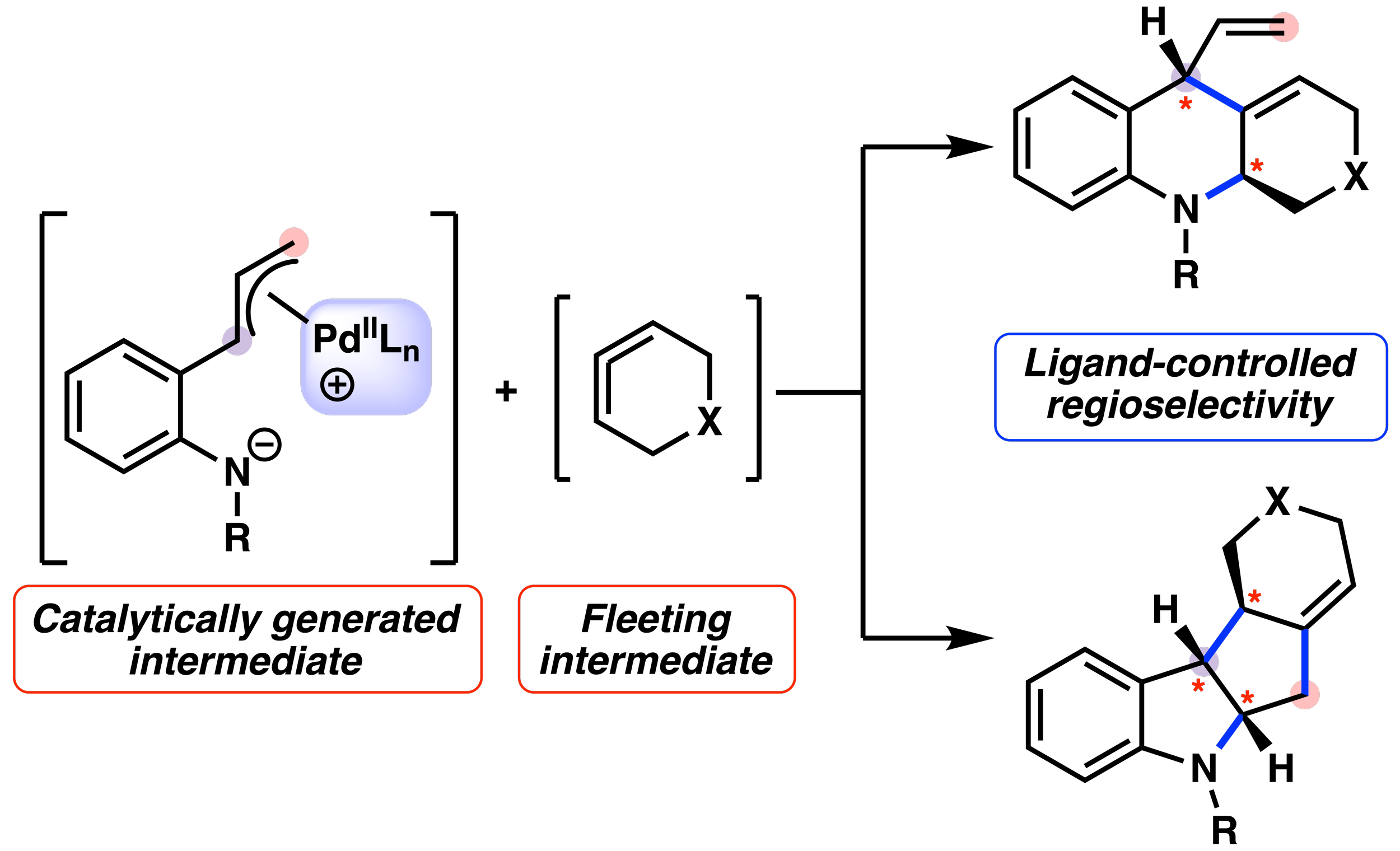
Read MoreCheck out our Catalyst-Controlled Annulations of Strained Cyclic Allenes with π-Allylpalladium Complexes published in ACS!
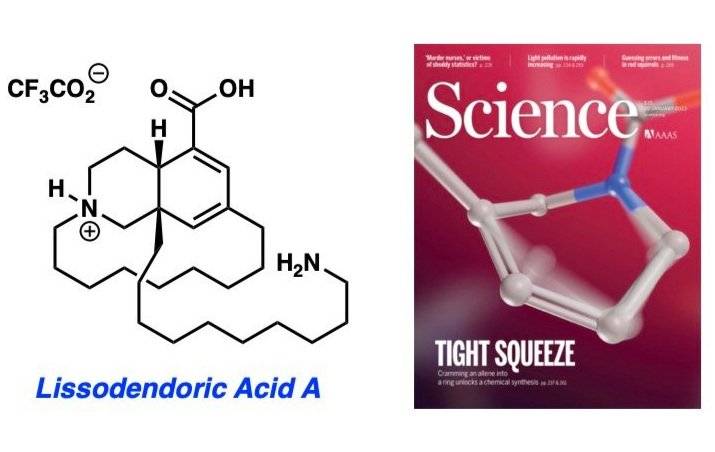
Read MoreCheck out our total synthesis of Lissodendoric Acid A published in Science and featured on the journal’s cover!
Read More8-Hydroxygeraniol is a key biosynthetic precursor to all monoterpene indole alkaloids. Check out our simple and scalable synthesis of 8-hydroxygeraniol published in Organic Syntheses!
Read MoreA rapidly growing area of research involves the manipulation of amides using nickel catalysis. These transformations are attractive due to nickel's high abundance, low cost, and minimal CO2 footprint. Check out our latest report published in Organic Syntheses on a scalable and practical Ni-catalyzed reduction of 12-aminododecanolactam!
Read MoreNi-catalyzed Suzuki–Miyaura cross-couplings of aliphatic amides provide a mild complement to traditional methods for the synthesis of alkyl–aryl ketones. Check out our report published in Organic Letters on a benchtop protocol that improves the practicality of these reactions using a paraffin-encapsulation strategy!
Read MoreStrained heterocyclic allenes represent a class of valuable intermediates that remain underdeveloped. Check out our synthesis of 3,4-oxacyclohexadiene precursors and studies on their utility in trapping reactions and enantiospecific Diels–Alder reactions published in ACIE.
Read More“Collaboration” is not the first word most associate with the field of total synthesis. Yet the complexity of natural products lends itself to advancements not only in synthetic chemistry, but in biocatalysis, biosynthesis, computational chemistry, and drug discovery as well. Here we highlight recent advances in these fields brought on by collaborations in the total synthesis of natural products.
Read MoreAn electron-rich benzylic secondary alcohol enables the reduction of a wide range of aromatic ketones under mildly basic conditions without the need for a transition or main group metal catalysts, with the potential for synthesis of enantioenriched products!
Read MoreUtilizing Ni/NHC–paraffin capsules developed by our laboratory and commercialized by TCI, undergraduates can now perform air/moisture-sensitive cross-couplings of amides in laboratory classes!
Read MoreOur collaboration with Codexis has been published in Nature’s new open access chemistry journal, Communications Chemistry. Check out our chemoenzymatic approach to enantioselective alcohol synthesis from amides using C–N bond activation!
Read MoreRapid access to diverse heterocyclic conjugated scaffolds via strained intermediates. Synthetic applications include donor–acceptor fluorophores as well as conjugated fluorescent oligomers!
Read MoreJason Chari’s collaboration with the Tang Lab has been published in JACS. Check out these efforts to uncover a new domain architecture among fungal PKSs!
Read MoreOur quick approach to synthesizing silyltriflate precursors to cyclohexyne and 1,2-cyclohexadiene has been published in JOC.
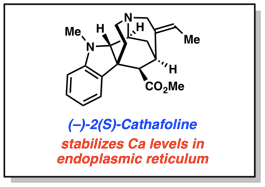
Read MoreCheck out our 100th publication! A collaboration involving nearly 100 co-authors, led by the NIH NCATS. It features 2(S)-cathafoline, a natural product our lab has synthesized.
Read More